
Rapid Reviews in Ireland
Systematic reviews have long been a key component of evidence-based medicine. The use of methods to expedite systematic reviews is ever-increasing due to time and resource constraints. Systematic reviews typically take at least 12 months to conduct compared to rapid reviews, which take a number of weeks. Hence, Rapid Reviews has become a pragmatic alternative to comprehensive systematic reviews. However, it is important that Rapid Review methods remain rigorous to support good policy development and decisions.
Rapid Reviews are particularly important in the field of health technology assessment (HTA), where they are streamlined assessments of healthcare technologies, interventions, or treatments used to support informed decision-making in a timely and resource-efficient manner. Rapid Review methods are not unique to HTA, although the need for timely evidence to underpin the assessment of new technologies makes them particularly relevant in this context.
The National Centre for Pharmacoeconomics (NCPE) is the Irish organisation responsible for conducting Rapid Reviews and HTAs related to pharmaceuticals. They evaluate the clinical and cost-effectiveness of healthcare interventions to inform decision-making regarding reimbursement with the Irish healthcare system. The objective of the Rapid Review is to determine whether a HTA is required or not for a pharmaceutical.
Rapid Reviews assessed by the NCPE involves a process that is set out in their updated Rapid Review template. The template, to be completed by the applicant, is intended to provide a summary of information to facilitate the Rapid Review process. Version 2.2 of this template was published on 05 August 2022 and replaces Version 2.1. Version 2.2 – (Rapid Review Template | National Centre for Pharmacoeconomics (ncpe.ie)) includes a requirement to submit the NCPE Budget Impact Model Template for all RR submissions and contains updated instructions regarding “academic-in-confidence” data.
The Rapid Review application is comprehensive and is set out in a systematic way under the following headings:
- Details of the Intervention
- The Disease and Place in Therapy
2.1. The disease/condition
2.2. Epidemiology of the disease in Ireland
2.3. Clinical guidelines and standard of care in Ireland
2.4. Place in therapy
2.5. Comparators - Clinical evidence
3.1. Study Design
3.2. Clinical efficacy
3.3. Clinical safety - Comparative costs
- Budget Impact Analysis
- International HTA
- References
- Appendices
Appendix 1: Clinical opinion
Appendix 2: Confidential information
References
As mentioned, every Rapid Review must now be accompanied by a Budget Impact Model Template. This is intended to guide Applicants in the calculation of Irish population estimates, patient population, drug acquisition costs, additional costs, cost offsets, results, presentation of budget impact results, and analysis of uncertainty. Version 1.8 of this template was published on 08 May 2023 and replaces Version 1.7.
Prior to starting the Rapid Review assessment, the NCPE Review Group will ensure that the Applicant submission includes:
- A completed Rapid Review Submission Template in both .docx and .pdf format, including all Appendices · a NCPE Budget Impact Model Template in .xlsm format using the standard NCPE template.
- Full-text copies of all references in .pdf format.
- a RIS file of all references.
- The draft SmPC, if the Rapid Review is submitted to the Committee for Medicinal Products for Human Use (CHMP)positive opinion, as the SmPC and the European Public Assessment Report (EPAR) will not be published.
- A copy of the EPAR from the reference country for products authorised via mutual recognition procedure.
If the submission is incomplete, the Applicant will be requested to submit the missing element(s). If errors are identified in the drug cost and/or budget impact calculations, the Applicant will be requested to correct the errors and resubmit the relevant files within three working days of the request. All electronic files must be submitted using a secure link provided by the NCPE.
Once all the information is received and the NCPE is happy to proceed the information from the applicant will be reviewed. Once this process is finished, there are 5 possible recommendations:
- A full Health Technology Assessment is recommended to assess the clinical effectiveness and cost-effectiveness of [Medicine] compared with the current standard of care (HTA recommended)
– A more detailed assessment i.e. HTA is required as some factors around the medication are unclear. It may be the case that it is not clear if the medicine is value for money.
- A full HTA is recommended to assess the clinical effectiveness and cost-effectiveness of [Medicine] compared with the current standard of care, on the basis of the proposed price relative to currently available therapies (HTA recommended at submitted price)
-The price of the medicine is higher than other treatments used for the particular condition, and it is not clear that the medicine is value for money. A full HTA assessment may not be needed if the HSE can agree on a suitable price reduction with the applicant.
- A full HTA is not recommended. The NCPE recommends that [Medicine] be considered for reimbursement (HTA not recommended)
-It is recommended that the HSE consider providing the medicine. The medicine is considered to possibly work as well or better than other ways to manage the condition and it is believed that the medicine is value for money. Therefore, a full HTA is not required.
- A full HTA is not recommended. The NCPE recommends that [Medicine] not be considered for reimbursement at the submitted price (HTA not recommended)
-It is recommended that the HSE not provide the medicine in question unless the HSE can agree on a suitable price reduction with the applicant. The price of the medicine is higher than other ways to manage the condition and is believed not to be value for money. Therefore, a full HTA is not recommended.
- A full HTA is not recommended until additional efficacy and/or safety data is submitted. On the basis of current evidence, the NCPE recommends that [Medicine] not be considered for reimbursement (HTA not recommended until further evidence)
-It is believed that a full HTA should not be done because there is not enough information on how well the medicine works. The medicine will be assessed again when the applicant can provide more information.
Please note that in a small number of cases, reimbursement not recommended can be an outcome of the Rapid Review process.
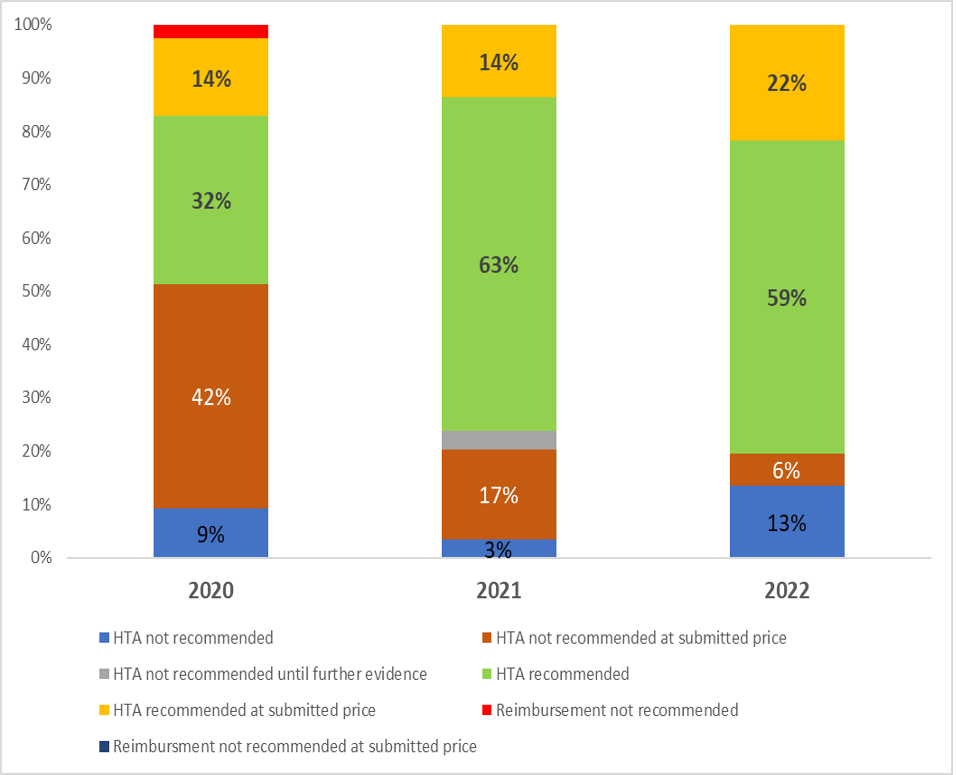
The graph below shows that 60% of new pharmaceuticals result in a HTA. A key feature of the Irish system is that it is possible to avoid a HTA following a price reduction. In 2022, 28% of Rapid Reviews involved price negotiations resulting in a HTA being avoided and more timely market access.
Outcomes from Rapid Reviews in Ireland
Whatever decision you encounter it is part of an overall reimbursement process which is set out below (this NCPE flowchart can be accessed on the NCPE website).
Reimbursement Process Flowchart
NCPE Stop-Clock Process
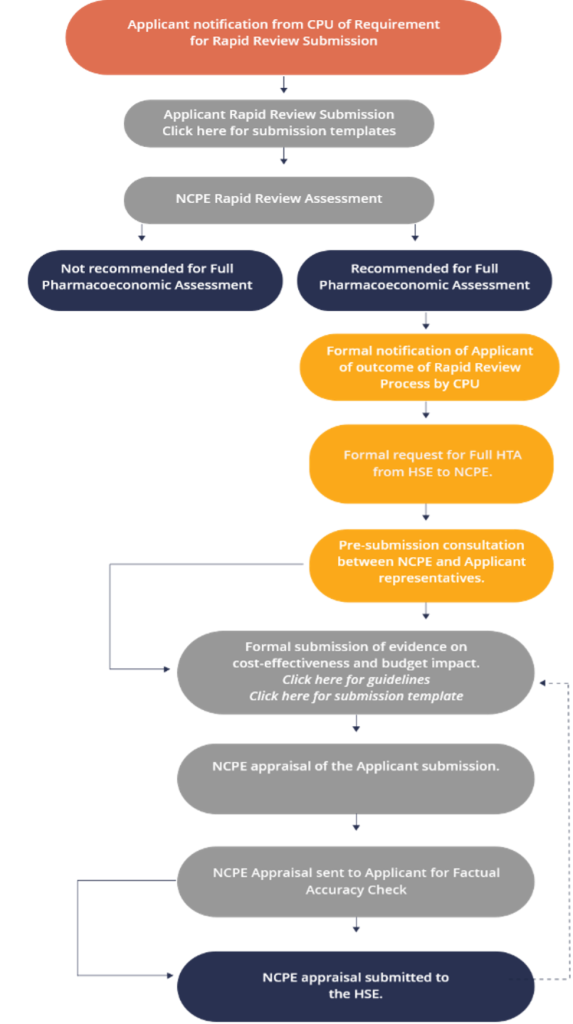
Summary of the health technology assessment process in Ireland, HSE-CPU – Health Service Executive Corporate Pharmaceutical Unit, HTA – health technology assessment, NCPE – National Centre for Pharmacoeconomics
Salutem Insights has created a short video to summarise the reimbursement process – Understanding the Irish Reimbursement Process for Medicines
If you are starting out on your reimbursement journey or have any questions about the process, please do not hesitate to contact us