Rapid Reviews in Ireland
Systematic reviews have long been a key component of evidence-based medicine. The use of methods to expedite systematic reviews is ever-increasing due to time and resource constraints. Systematic reviews typically take at least 12 months to conduct compared to rapid reviews, which take a number of weeks. Hence, Rapid Reviews has become a pragmatic alternative to comprehensive systematic reviews. However, it is important that Rapid Review methods remain rigorous to support good policy development and decisions.
Rapid Reviews are particularly important in the field of health technology assessment (HTA), where they are streamlined assessments of healthcare technologies, interventions, or treatments used to support informed decision-making in a timely and resource-efficient manner. Rapid Review methods are not unique to HTA, although the need for timely evidence to underpin the assessment of new technologies makes them particularly relevant in this context.
The National Centre for Pharmacoeconomics (NCPE) is the Irish organisation responsible for conducting Rapid Reviews and HTAs related to pharmaceuticals. They evaluate the clinical and cost-effectiveness of healthcare interventions to inform decision-making regarding reimbursement with the Irish healthcare system. The objective of the Rapid Review is to determine whether a HTA is required or not for a pharmaceutical.
Rapid Reviews assessed by the NCPE involves a process that is set out in their updated Rapid Review template. The template, to be completed by the applicant, is intended to provide a summary of information to facilitate the Rapid Review process. Version 2.2 of this template was published on 05 August 2022 and replaces Version 2.1. Version 2.2 – (Rapid Review Template | National Centre for Pharmacoeconomics (ncpe.ie)) includes a requirement to submit the NCPE Budget Impact Model Template for all RR submissions and contains updated instructions regarding “academic-in-confidence” data.
The Rapid Review application is comprehensive and is set out in a systematic way under the following headings:
- Details of the Intervention
- The Disease and Place in Therapy
2.1. The disease/condition
2.2. Epidemiology of the disease in Ireland
2.3. Clinical guidelines and standard of care in Ireland
2.4. Place in therapy
2.5. Comparators - Clinical evidence
3.1. Study Design
3.2. Clinical efficacy
3.3. Clinical safety - Comparative costs
- Budget Impact Analysis
- International HTA
- References
- Appendices
Appendix 1: Clinical opinion
Appendix 2: Confidential information
References
As mentioned, every Rapid Review must now be accompanied by a Budget Impact Model Template. This is intended to guide Applicants in the calculation of Irish population estimates, patient population, drug acquisition costs, additional costs, cost offsets, results, presentation of budget impact results, and analysis of uncertainty. Version 1.8 of this template was published on 08 May 2023 and replaces Version 1.7.
Prior to starting the Rapid Review assessment, the NCPE Review Group will ensure that the Applicant submission includes:
- A completed Rapid Review Submission Template in both .docx and .pdf format, including all Appendices · a NCPE Budget Impact Model Template in .xlsm format using the standard NCPE template.
- Full-text copies of all references in .pdf format.
- a RIS file of all references.
- The draft SmPC, if the Rapid Review is submitted to the Committee for Medicinal Products for Human Use (CHMP)positive opinion, as the SmPC and the European Public Assessment Report (EPAR) will not be published.
- A copy of the EPAR from the reference country for products authorised via mutual recognition procedure.
If the submission is incomplete, the Applicant will be requested to submit the missing element(s). If errors are identified in the drug cost and/or budget impact calculations, the Applicant will be requested to correct the errors and resubmit the relevant files within three working days of the request. All electronic files must be submitted using a secure link provided by the NCPE.
Once all the information is received and the NCPE is happy to proceed the information from the applicant will be reviewed. Once this process is finished, there are 5 possible recommendations:
- A full Health Technology Assessment is recommended to assess the clinical effectiveness and cost-effectiveness of [Medicine] compared with the current standard of care (HTA recommended)
– A more detailed assessment i.e. HTA is required as some factors around the medication are unclear. It may be the case that it is not clear if the medicine is value for money.
- A full HTA is recommended to assess the clinical effectiveness and cost-effectiveness of [Medicine] compared with the current standard of care, on the basis of the proposed price relative to currently available therapies (HTA recommended at submitted price)
-The price of the medicine is higher than other treatments used for the particular condition, and it is not clear that the medicine is value for money. A full HTA assessment may not be needed if the HSE can agree on a suitable price reduction with the applicant.
- A full HTA is not recommended. The NCPE recommends that [Medicine] be considered for reimbursement (HTA not recommended)
-It is recommended that the HSE consider providing the medicine. The medicine is considered to possibly work as well or better than other ways to manage the condition and it is believed that the medicine is value for money. Therefore, a full HTA is not required.
- A full HTA is not recommended. The NCPE recommends that [Medicine] not be considered for reimbursement at the submitted price (HTA not recommended)
-It is recommended that the HSE not provide the medicine in question unless the HSE can agree on a suitable price reduction with the applicant. The price of the medicine is higher than other ways to manage the condition and is believed not to be value for money. Therefore, a full HTA is not recommended.
- A full HTA is not recommended until additional efficacy and/or safety data is submitted. On the basis of current evidence, the NCPE recommends that [Medicine] not be considered for reimbursement (HTA not recommended until further evidence)
-It is believed that a full HTA should not be done because there is not enough information on how well the medicine works. The medicine will be assessed again when the applicant can provide more information.
Please note that in a small number of cases, reimbursement not recommended can be an outcome of the Rapid Review process.
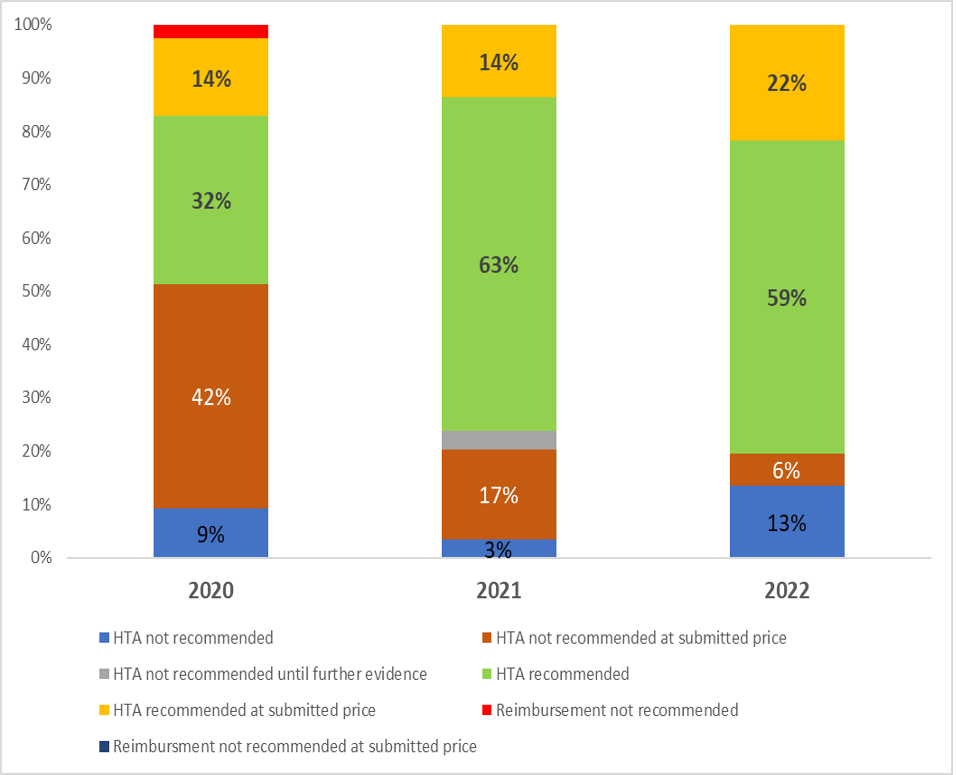
The graph below shows that 60% of new pharmaceuticals result in a HTA. A key feature of the Irish system is that it is possible to avoid a HTA following a price reduction. In 2022, 28% of Rapid Reviews involved price negotiations resulting in a HTA being avoided and more timely market access.
Outcomes from Rapid Reviews in Ireland
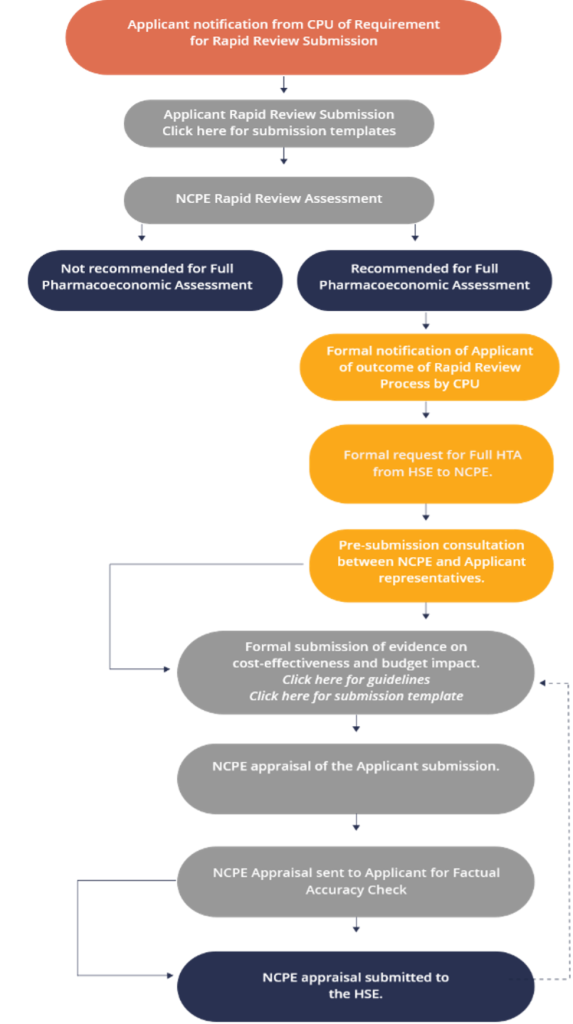
Whatever decision you encounter it is part of an overall reimbursement process which is set out below (this NCPE flowchart can be accessed on the NCPE website).
Reimbursement Process Flowchart
NCPE Stop-Clock Process
Summary of the health technology assessment process in Ireland, HSE-CPU – Health Service Executive Corporate Pharmaceutical Unit, HTA – health technology assessment, NCPE – National Centre for Pharmacoeconomics
Salutem Insights has created a short video to summarise the reimbursement process – Understanding the Irish Reimbursement Process for Medicines
If you are starting out on your reimbursement journey or have any questions about the process, please do not hesitate to contact us
Health Technology Assessments (HTA’s)
Health technology assessment (HTA) is a multidisciplinary process that evaluates the clinical effectiveness, safety, economic viability, and broader impact of health technologies. Health technologies refer to any intervention, such as medicines, vaccines, medical devices, procedures, and systems, used to prevent, diagnose, or treat a disease or condition that affects human health. HTAs are essential to ensure that technologies being considered for adoption are safe, effective, and can deliver the desired outcomes at a reasonable cost. By assessing the technological properties of health technologies, HTA also help healthcare systems identify promising innovations that can improve health outcomes and enhance patient care.
In a rapidly evolving healthcare landscape, HTA evaluation plays a crucial role in informing policy decision-makers, healthcare providers, and patients about the most effective and cost-efficient technologies to adopt. HTAs must be conducted in a structured and thorough manner. Therefore, the HTA process involves several steps, including systematic reviews, a review of the clinical evidence, economic evaluation, and budget impact estimation. This information should be unbiased and evidence based.
What is the basis for HTA in Ireland?
The Health Service Executive (HSE) has statutory responsibility for decisions on pricing and reimbursement of medicines in accordance with the 2013 Health Act. As part of this statutory assessment process the HSE must consider the affordability of each individual decision against overall resources as allocated. In line with the 2013 Health Act, if a Company would like a medicine to be reimbursed by the HSE the Company must first submit an application to the HSE.
In reaching its decision on the whether to include a new medicine on the reimbursement list, Part 3 of the 2013 Health Act requires the HSE to have regard to the following criteria:
- The health needs of the public
- The cost-effectiveness of meeting health needs by supplying the item concerned rather than providing other health services [2]
- The availability and suitability of items for supply or reimbursement
- The proposed costs, benefits and risks of the item or listed item relative to therapeutically similar items or listed items provided in other health service settings and the level of certainty in relation to the evidence of those costs, benefits, and risks [4]
- The potential or actual budget impact of the item or listed item [5]
- The clinical need for the item or listed item
- The appropriate level of clinical supervision required in relation to the item to ensure patient safety
- The efficacy (performance in trial), effectiveness (performance in real situations) and added therapeutic benefit against existing standards of treatment (how much better it treats a condition than existing therapies)
- The resources available to the HSE.
Three of the above criteria are included in a HTA: comparative clinical effectiveness (4), cost effectiveness (2) and budget impact (5).
In submitting an application for reimbursement, a Company will propose a price for reimbursement having regard, as applicable, to this Framework Agreement 2021 (an agreement between the Irish Pharmaceutical Healthcare Association (IPHA) and the HSE regarding the pricing and supply of medicines.
What qualifies as a health technology?
The term ‘health technology’ encompasses a wide range of health interventions. ‘Technology’ includes any intervention that may be used to promote health; to prevent, diagnose or treat a disease; or in rehabilitation or long-term care.
Technologies include, but are not limited to:
- Medicines, including vaccines
- Medical devices, including equipment such as surgical robots and implantable devices
- Diagnostic tests, for example, tests to detect colorectal cancer
- Surgical procedures, such as coronary artery bypass
- Public health interventions, for example, a colorectal cancer screening programme
- Support systems, such as electronic patient records
- Organisational features, for example, the establishment of centres of excellence.
A health technology does not have to be a medical intervention given directly to a patient. It may be as broad as a restructuring of how an aspect of the health service is organised and delivered, such as the transfer of a service from secondary to primary care settings. The technology does not need to be new, and a HTA can look at changing existing services through reallocation of resources or through standardising current services. A HTA can also be used to inform decisions about discontinuing ineffective technologies and, thereby, support the best use of available resources.
Who carries out HTAs in Ireland?
HTAs are carried out by a range of public bodies and research groups.
- The Health Information and Quality Authority (HIQA) is mandated under the Health Act to carry out HTAs and develop guidelines for HTA preparation across the Irish health system (this includes pharmaceuticals, devices, diagnostics, procedures, care pathways and public health activities). They also undertake HTAs to inform national-level policy decisions and national health service decisions.
- The National Centre for Pharmacoeconomics (NCPE) is commissioned by Corporate Pharmaceutical Unit of the Health Service Executive (HSE-CPU) to perform HTAs on pharmaceutical products, in particular on whether new treatments should be made available at the state’s expense. All new medicines are required to undergo a rapid review (RR) appraisal by the NCPE. Following this, high-cost medicines or those predicted to have a significant budget impact then undergo a full HTA appraisal by the NCPE. The NCPE also work with other HTA bodies such as HIQA as well as engaging in research with academic units.
- The HSE assesses the clinical and cost-effectiveness of medical devices being introduced to the market.
- The pharmaceutical industry undertakes HTAs in support of applications to have products reimbursed through the Community Medicines Schemes and hospitals. These are appraised by the NCPE.
- Academic groups undertake HTAs on behalf of the healthcare industry and for research purposes.
What is evaluated in a HTA?
The content of a HTA will vary depending on the decision being supported. For example, a full HTA to support a national decision will typically collect and summarise evidence under a number of headings. These headings include:
- Description of the technology – what it is used for and how it works.
- Burden of disease – who gets the disease and what are the typical outcomes.
- Clinical effectiveness and safety –this includes data form clinical trials, real-world studies and systematic reviews
- Cost-effectiveness and budget impact – the economic evaluation is a crucial component of the HTA. It analyses and evaluates the costs and benefits associated with the intervention and helps determine whether the intervention provides value for money in relation to its health outcomes. The financial impact is also assessed and involves the costs associated with the implementing and delivering the intervention and its potential impact on the overall healthcare budget.
- Organisational and social aspects – how does the technology impact on patients and the organisation of services.
- Ethical and legal issues – HTA considers ethical and societal considerations associated with the intervention. This may include evaluating issues such as equity of access, patient preferences, impact on vulnerable populations, and the broader societal implications of adopting the intervention.
There are recommended guidelines around the evidence required under these particular headings. Each of these areas is important. For ease of use, the national guidelines have been developed as stand-alone documents.
Guidelines for Economic Evaluation of Health Technologies in Ireland
Guidelines for Evaluating the Clinical Effectiveness of Health Technologies in Ireland
Guidelines for the Budget Impact Analysis of Health Technologies in Ireland
The guidelines are intended to be applicable to all healthcare technologies, including pharmaceuticals, procedures, medical devices, broader public health interventions and service delivery models. It is important to refer to the most up-to-date version of these guidelines.
The HTA Process
A summary of the HTA process from the NCPE website is outlined in Fig. 1 and the text below. The formal reimbursement process begins once the applicant company receives a positive decision from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) and they have certainty around the reimbursement price for which they intend to apply.
Fig. 1
Summary of the health technology assessment process in Ireland, HSE–CPU – Health Service Executive Corporate Pharmaceutical Unit, HTA – health technology assessment, NCPE – National Centre for Pharmacoeconomics,
The HTA process, following CHMP opinion, consists of several steps:
- The company makes a Rapid Review (RR) submission to the NCPE in accordance with NCPE requirements. Each submission pertains to one medicine for one indication.
- The NCPE review group appraises the RR submission within a 4-week timeframe. The decision criteria considered by the NCPE during the RR appraisal includes eligible patient population, robustness of clinical evidence, cost of the medicine relative to current standard of care and budget impact.
- The NCPE informs the HSE-CPU as to whether an HTA is required and notifies the applicant company when the RR is completed, and that the outcome will be posted on the NCPE website.
- Where an HTA has been deemed necessary, a pre-submission consultation is arranged between the NCPE and the applicant company. The pre-submission meeting provides an opportunity for the applicant company and the NCPE to discuss what will be included in the HTA submission.
- The applicant company produces an HTA submission in accordance with NCPE requirements and HIQA guidelines.
- The NCPE may seek clarification from the applicant company on any aspect of the submission. Preliminary questions are sent to the applicant company once the NCPE have conducted a thorough review of the submission. The applicant company is requested to respond to the questions within 1 month.
- The appraisal of the HTA should take a maximum of 90 days, excluding the time taken by the applicant company to prepare the submission and to make any required amendments. A draft appraisal report is sent back to the applicant company for a factual accuracy check, which should be carried out within 5 working days.
- The final appraisal report is produced. Where cost effectiveness has been demonstrated, a positive reimbursement recommendation is made. If the medicine is not considered cost effective at the submitted price, this is specified. If it is not considered cost effective because of unresolved concerns about the validity of any aspect of the submission, this is detailed. Further information on the updated NCPE recommendations can be found on the NCPE website.
- The final appraisal report is sent to the HSE-CPU. The HSE considers the appraisal report in conjunction with the additional criteria set out in the Health Act 2013.
- For certain medicines, a final decision can be reached, and the applicant company notified. In most cases, the medicine will be considered by the HSE Drugs Group, who will make a reimbursement recommendation to the HSE Leadership Team, who in turn make the reimbursement decision.
Salutem Insights have created a short video to summarise the reimbursement process here : Understanding the Irish Reimbursement Process for Medicines
It is important to note that the HTA process in Ireland is continually evolving, and the specific steps and bodies involved may vary depending on the specific intervention and circumstances. However, the overall aim remains to ensure that healthcare technologies are evaluated in a rigorous, transparent, evidence-based and equitable manner to facilitate informed decision-making and optimise healthcare resource allocation in Ireland.
Timelines
From the Salutem Insights database the timeframe between RR submission and reimbursement has varied over time for new medicines that have undergone a HTA (see Fig. 2). For example, in 2010, the average time between RR submission and reimbursement was 290 days compared to 829 days in 2022. These timelines do not take into account the NCPE stop-clock process and the time it takes to submit a HTA to the NCPE following the outcome from the RR.
Fig. 2
Salutem Insights and HTAs
Salutem Insights have been conducting HTAs in Ireland since 2007. They have conducted HTAs on vaccines, orphan medicines, oncology medicines, orphan medicines and gene therapies. Salutem Insights can help you navigate the reimbursement process in Ireland with ease.
If you have any questions or need further insights, please contact Sandra at sandraredmond@saluteminsights.com or email info@saluteminsights.com or contact us here